Lesson Plan: Long-term Biochemical Experimentation
Print version of this lesson plan.
Egg Shell and Vinegar
Subjects
Earth Science, Ecology
Grade-level
Elementary, 2-5
Middle School, 6-8
Time required
60 minutes for 3 or more days
(Additional time is required for homework assignments and assessment tools.)
Materials required
- 3 Flasks (or a clear glass or plastic cup)
- 3 Hard boiled eggs
- Water
- Vinegar
- Camera/video (optional)
- pH meter
Content Standards
This lesson fulfills various aspects of the following Guam Department of Education Standards depending on grade level: 1st through 3rd and 7th grades.
Lesson Plan
Part I: Experiment- Egg Shell
Description
Simple chemistry experiments will be conducting investigating the effects of water and vinegar on egg shell.
Emphasis
Learning about the chemistry that surrounds us.
Objectives
- Students will identify that egg shell behaves differently in water and vinegar.
- Students will be able to describe the chemical reaction observed when hard boiled eggs are added to vinegar.
- Students will be able to articulate what they observed in the chemical reaction.
Procedure
Teacher Notes
This experiment will require students to fill out the “Egg Shell Experiment Worksheet.” A copy can be downloaded with lesson plan.
Activity
- Have students note the appearance of the egg.
- Is it hard, soft, smooth, rough, squishy?
- Label one cup “water”, label the other “vinegar”.
- Place one egg in the water cup, place the other egg in the vinegar cup, leave the third egg in a empty container.
- Fill each cup with the appropriate liquid (to within 1/2” from the top).
- Have students use the Egg Shell Experiment Worksheet to note their observations of each cup.
- Encourage students to be very detailed.
- At each time point, students can take the egg out and feel the surface.
- Take pictures and/or video for record keeping in addition to Egg Shell Experiment Worksheet.
- Compare the egg shell in water or vinegar to the non-submerged egg; do they notice any differences?
- After at least eight hours* , students you notice that the hard shell shell has been broken down and is now soft.
(* Note: This experiment can be conducted over several days.)
Teacher Notes
The vinegar has reacted with the calcium carbonate in the egg shell and broken it down in to water (H20) and carbon dioxide (CO2).
Refer to the chemical reaction below:
CaCO3 (calcium carbonate) + CH3COOH (vinegar) = CO2 (carbon dioxide) + H20 (water) + Ca
- This is a good opportunity to discuss how and why molecular formulas are written as such.
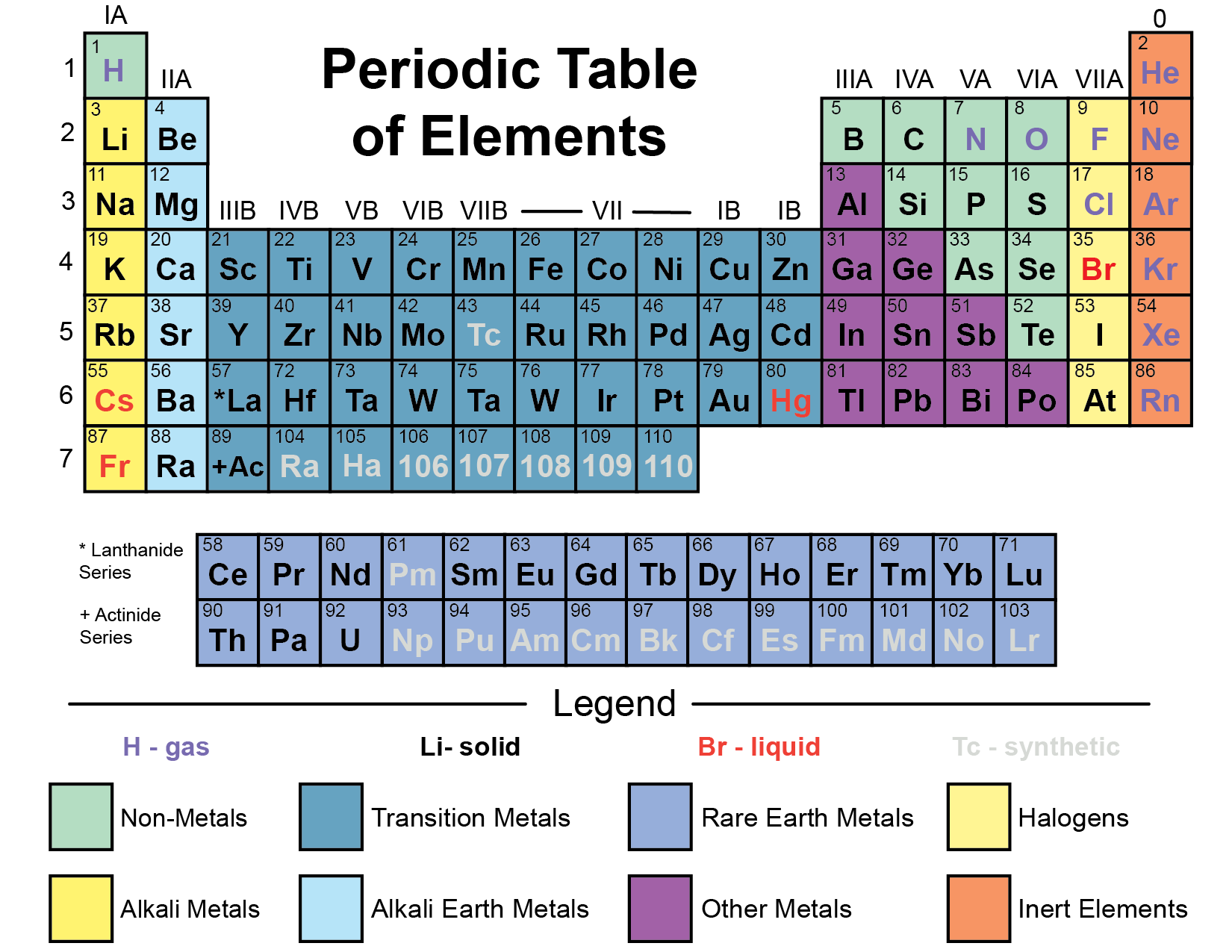
Periodic Table of Elements
Refer to the periodic table located below.
All atoms in the universe are abbreviated with either one capital letter (oxygen = O, hydrogen = H, carbon = C, nitrogen = N, ) or one capital letter followed by a lower case letter (calcium = Ca, Helium = He, Sodium = Na, Chloride = Cl). Next to each letter, or set of letters, is usually a number, the number tells you how many of that particular atom are present in any given molecule (H2= 2 atoms of hydrogen). If a number appears in front of the molecule or atom, it means that there are two of that particular molecule or atom. So to be technically correct we should write the aforementioned chemical reaction like this:
CaCO3 + 2(CH3COOH) = CO2 + H20
- You need at least two molecules of vinegar for this chemical reaction to occur. Typically, when we perform this chemical reaction in the classroom we are using millions of molecules of vinegar and therefore this isn’t a concern.
Some more examples of this are:
- 2(CaCO3) = two molecules of calcium carbonate
- 2(H20) = two molecules of water
- 24(NaCl) = Twenty-four molecules of sodium chloride (salt!)
- 6(CO2) = six molecules of carbon dioxide
Part II: Go Further- pH Experiment
Note: Appropriate for advanced 5th grade classes.
Materials required
- pH meter
Objective
Do the following experiment to learn more about pH and how it can change, record your results in Table 1.3.
Emphasis
Chemical compounds can have an effect on the pH of a solution.
Questions or Assessment
What is pH?
pH is a measure of the number of hydrogen atoms in a solution. The more hydrogen atoms in a solution, the lower the pH is. Conversely, the less hydrogen atoms that are present the higher the pH. pH can range from 0 to 12. Hydrochloric acid is a very strong acid and has a pH of 1. Acetic acid (also known as VINEGAR!) has a pH of 2.4. While vinegar is still a strong acid, one could say that it is less potent that hydrochloric acid. It is easy to measure the pH, using a pH meter. the pH meter measured the number of hydrogens in any given solution.
Procedure
- Measure the pH of the water.
- Measure the pH of the vinegar.
- Place an egg in each flask.
- Measure the pH of the water and vinegar again.
- Leave the eggs in the flasks and measure the pH every 24 hours for three days.
- What changes did you observe, if any?
- Did the egg behave differently in the water compared to the vinegar?
- Can you make any conclusions about the effect that calcium carbonate had on the pH of the vinegar and the water?
- In which solution did the pH change? Why?
- Compare the surface of the egg shell in water or vinegar to the one in the empty container. What differences do you notice?
Table 1.3
| Table 1.3 | pH (no egg) | pH (plus egg) | Day 1 pH + 24 hrs | Day 2 pH + 48 hrs | Day 3 pH + 96 hrs |
|---|---|---|---|---|---|
| Water | |||||
| Vinegar |
Egg Shell Experimentation Worksheet
Editor’s note: Funding for this lesson plan was provided by the Howard Hughes Medical Institute, Olivera Lab, University of Utah. Please email Laura Biggs, PhD at [email protected] with questions or comments.